

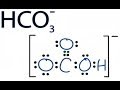















The conjugate base of bicarbonate, HCO 3- is carbonate, CO3 2-.. HCO3- is a conjugate acid, H 2 CO 3 The conjugate acid and conjugate base of bicarbonate ion, HCO3 -, are, respectively: a) H3O+ and OH- b) ) H2CO3 and CO3 2- e) CO32- and OH- Click here👆to get an answer to your question ️ 13.(i)What will be the conjugate bases for the following Bronsted acids? HF, H2SO4, HCO3, H3PO4 (ii) What will be the conjugate acids for the following Bronsted bases? NH 2, NH3, HCOO\", CIO, Finding conjugate acid and conj. base of HCO3- Post by ttay190190 » Sat Dec 07, 2013 8:24 pm In parts (c) and (d) of problem 11.1 from the textbook, we are asked to write the formula of the conjugate base of HCO3- (book answer: H2CO3) and the conjugate acid of HCO3- (book answer: CO3^2-). The conjugate base of HCO3- is CO32-. Conjugates always differ by one H+. A conjugate base has one fewer H+, while a conjugate acid has one more H+. 1) What is the conjugate base of HCO3−? Express your answer as a chemical formula. 2) What is the conjugate acid of HPO32− ? Express your answer as a chemical formula. 3) Among three bases, X−, Y−, and Z−, the strongest one is Y−, and the weakest one is Z−. Rank their conjugate acids, HX, HY, and HZ, in order of decreasing strength. ACID + BASE = CONJUGATE BASE + CONJUGATE ACID 1.Acid after losing a proton becomes conjugate base. 2. Base after accepting a proton becomes conjugate acid. To find conjugate acid, HCO3- must act like a base. ANSWER is H2CO3 Answer to Part B What is the conjugate base of HCO3 ? Express your answer as a chemical formula. View Available Hint(s) - AED O ? The conjugate base is a substance formed when an acid loses a hydrogen ion. thus the conjugate base of any compound is the compound after the removal of H+ from them respectively. The conjugate base of acid HCO3- is (CO3)^2-The conjugate base of H2O is OH- Solved: What is the conjugate base of HCO3-? By signing up, you'll get thousands of step-by-step solutions to your homework questions. You can also...
[index] [6945] [4713] [8804] [2457] [261] [3228] [1585] [7715] [9818] [6040]
Use Bronsted Lowry Acid/Base Theory to identify conjugate acid base pairs.More free chemistry help at www.chemistnate.com To make a carbonate buffer of pH 10.00, how many grams of sodium carbonate (Na2CO3) must you add to 1.5 L of freshly prepared 0.20 M sodium bicarbonate to ma... About Press Copyright Contact us Creators Advertise Developers Terms Privacy Policy & Safety How YouTube works Test new features Press Copyright Contact us Creators ... About Press Copyright Contact us Creators Advertise Developers Terms Privacy Policy & Safety How YouTube works Test new features Press Copyright Contact us Creators ... Conjugate Acid Base Pairs, Arrhenius, Bronsted Lowry and Lewis Definition - Chemistry - Duration: 11:37. The Organic Chemistry Tutor 192,432 views. 11:37. 🚀To book a personalized 1-on-1 tutoring session:👉Janine The Tutorhttps://janinethetutor.com🚀More proven OneClass Services you might be interested in:👉One... A step-by-step explanation of how to draw the HCO3- Lewis Dot Structure (Hydrogen Carbonate or Bicarbonate Ion).For the HCO3- structure use the periodic tabl... Trick to Find Conjugate Acid and Conjugate Base / Ionic Equilibrium Tricks This chemistry video tutorial explains the concept of acids and bases using the arrhenius definition, bronsted - lowry and lewis acid base definition. It al...
Copyright © 2024 top100.playbestrealmoneygame.xyz